On June 7, 2021, the U.S. based BioTech company Biogen and Japanese pharmaceutical company Eisai have gained approval from the U.S. Food and Drug Administration (FDA) for the first and only drug to treat Alzheimer’s Disease after a long waited 115 years.
ADUHELM™ (aducanumab-avwa) is a beta-amyloid-directed antibody that targets the beta-amyloid plaque in the brain and disposes insoluble fibrils into it to break it down.
Beta-amyloid is formed from the breakdown of larger protein molecules (amyloid precursor protein), and the most toxic form is the beta-amyloid 42. If the protein is not cleared completely, the build-up can result in forming a toxic plaque that ends up triggering the neurodegenerative process, causing memory and cognitive loss.
Biogen has not disclosed the price of their drug, however many speculate that the year-long treatment cost will be placed between US$30,000- $56,000.
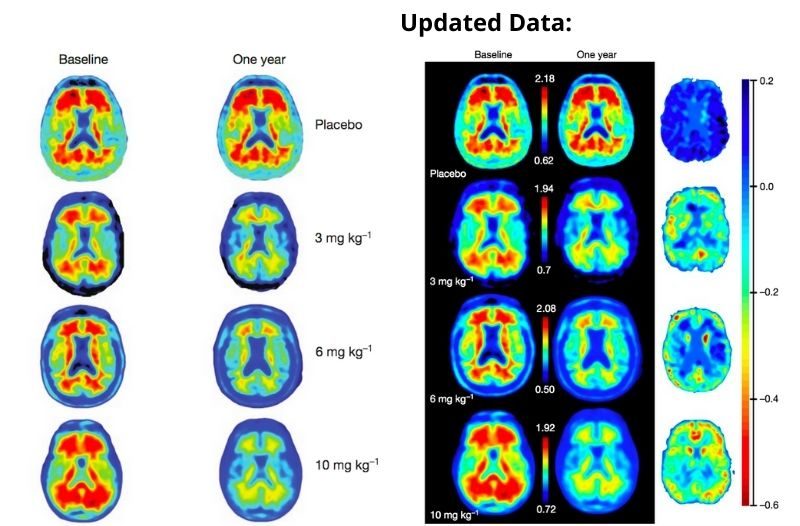
The approval of this drug was not easy, as it faced many controversial debates on its effectiveness after years of failed trials. Biogen discontinued two studies in 2019, after showing multiple disappointing data results of failed attempts to decrease the size of the beta-amyloid plaque in 1,748 patients. These results even had many experts debate on whether or not the beta-amyloid plaque was the root cause of Alzheimer’s.
A couple of months later, after thoroughly analysing more data, Biogen and Eisai published a new study with 3,285 patients (2,066 of them receiving the 18-month treatment course) and found promising results for those who received a higher dosage.
An expert panel then reconvened later in November 2020 to unanimously agreed for Biogen and Eisai to re-conduct an additional placebo-controlled study, after claiming that the evidence presented was not enough to prove the drug’s effectiveness towards memory and cognitive improvement. The decision was brought up to the FDA to decide whether or not they would want to see more result or agree to the success stories made by the patients. The committee’s decision was later extended from last March to June 7.
The FDA then reported back after thoroughly examining the clinical findings closely
“Although the Aduhelm data are complicated with respect to its clinical benefits, FDA has determined that there is substantial evidence that Aduhelm reduces amyloid-beta plaques in the brain and that the reduction in these plaques is reasonably likely to predict important benefits to patients,” said Dr Patrizia Cavazzoni, Director of the FDA Center for Drug Evaluation and Research.
The Alzheimer’s Association quickly showed its support toward’s the FDA’s decision, realising that although this may not be a cure, it is the first small victory to be celebrated.
Even with the approval, the FDA granted Aduhelm with a Fast Track designation, which means that Biogen will have to complete post-approval studies (phase 4 confirmatory trials) before marketing it, and failure to comply will result in the FDA’s withdraw of approval. The trial was only done on patients with mild cognitive degeneration therefore, it is important to doctors and patients to understand the extent of what Aduhelm can do, making sure to monitor patients closely for side effects like brain inflammation.
Related Articles





