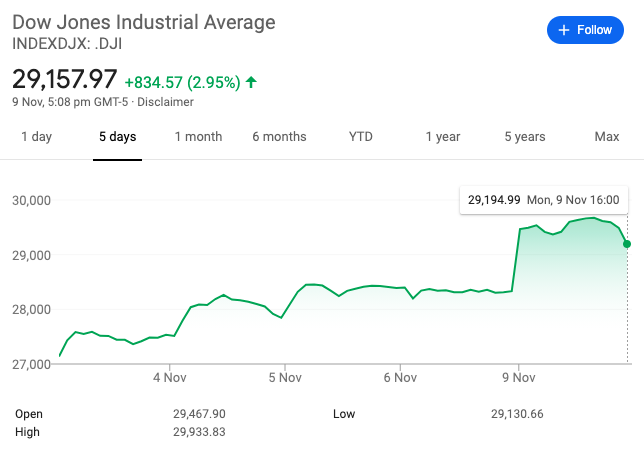
Stock markets surged on Monday after multinational pharmaceutical Pfizer Inc. announced its COVID-19 vaccine had a 90% success rate in test participants who had no prior history of being infected by the virus.
Why it matters: Pfizer is the first pharmaceutical company to announce positive results from a late-stage COVID-19 vaccine trial, and stock market surges reflected strong investor optimism.
• The Dow Jones Industrial Average closed at 29,157.97 (up 2.95%) on Monday after Pfizer’s announcement. The S&P 500 gained 1.2% Monday, close to a high achieved in early September.
• Shares of companies that stand to gain most from the return of normal activity — like airlines and shopping mall operators — soared, while those whose businesses were well suited under lockdowns were sharply lower.
• Shares of movie theater chain AMC skyrocketed 60% while concert promoter Live Nation rose more than 20%. Large retailers also benefited from the announcement – the SPDR S&P Retail ETF, which contains Gap, Best Buy, Target, Costco, and several other big chains, was up 2%.
• Video conferencing company Zoom suffered a drop of almost nearly 15%, while Netflix and Amazon suffered drops of 8.5% and 5%, respectively, as investors seek to prepare for a world returning back to normal.
The vaccine: Pfizer projects that it will produce up to 50 million doses of the vaccine by the end of this year, as well as 1.3 billion doses by the end of 2021.
• Its 44,000-person clinical trial was conducted in tandem with German biotech company BioNTech. So far, 94 participants (who were not previously infected) have contracted COVID-19. Of those 94 cases, fewer than 9 people were among those who received two shots of the vaccine, a strong indicator of the effectiveness of the two-dose vaccine.
• Professor Ugur Sahin, BioNTech co-founder and CEO, said that the trial would continue until a total of 164 COVID-19 cases have been confirmed. A final analysis will be conducted afterwards. Pfizer and BioNTech estimate that more safety data will be available by the third week of November.
• The study defined a case as a person with a positive test result and at least one COVID-19 symptom. Pfizer said the trial was evaluated by an external, independent Data Monitoring Committee.
• Speaking to The Washington Post, Dr Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, praised the results of the trial, saying “The results are really quite good, I mean extraordinary.”
Looking ahead: Competing in a race against time, several other companies are also developing COVID-19 vaccines.
• Global biotechnology company CSL will begin manufacturing at least 30 million vials of a COVID-19 vaccine jointly developed by British-Swedish pharmaceutical company AstraZeneca and scientists from Oxford University, the Sydney Morning Herald reported on Sunday.
• Manufacturing began on Monday in Melbourne, but the vaccine still needs to be approved by Australia’s Therapeutic Goods Association. Phase Three clinical trials are expected to wrap up by the end of the year.
• Australian Health Minister Greg Hunt predicted that the vaccine would be available to the public in March.
• Additionally, Moderna, a biotechnology firm, said that it expected interim findings from its study this month. Dr Fauci added that Pfizer’s results might bode well for the COVID-19 vaccine being developed by Moderna, commenting that “[Pfizer’s vaccine] gives you hope we might even have two vaccines.”
• Moderna is preparing to distribute 20 million doses of its vaccine, known as mRNA-1273, by the end of this year, and estimates that it will produce between 500 million and 1 billion doses in 2021.
Related Articles
US Elections: What Biden’s Win Means for Asia Pacific
What Ant’s IPO Suspension Means for Future FinTech Growth
Japan Removes Travel Ban on 9 Countries to Revive Battered Economy